| Ala | |
| A | -62.99 | -40.62 | 180.00 |
| C | -81.98 | -6.08 | 180.00 |
| B | -129.24 | 141.62 | 180.00 |
| P | -67.56 | 138.13 | 180.00 |
| G | 69.33 | 27.67 | 180.00 |
| E | 124.29 | -180.07 | 180.00 |
| |
|
| Cys | |
| A | -66.57 | -41.49 | 180.00 |
| C | -86.18 | -6.86 | 180.00 |
| B | -122.25 | 142.37 | 180.00 |
| P | -71.68 | 137.12 | 180.00 |
| G | 71.00 | 31.75 | 180.00 |
| E | 159.50 | -182.00 | 180.00 |
| |
|
| Asp | |
| A | -66.54 | -41.22 | 180.00 |
| C | -90.16 | 1.11 | 180.00 |
| B | -119.50 | 129.16 | 180.00 |
| P | -71.75 | 131.32 | 180.00 |
| G | 60.50 | 38.67 | 180.00 |
| E | 98.50 | -177.20 | 180.00 |
| O | -99.00 | 210.00 | 0.00 |
|
| Glu | |
| A | -65.63 | -40.90 | 180.00 |
| C | -83.35 | -6.32 | 180.00 |
| B | -120.71 | 136.01 | 180.00 |
| P | -67.76 | 132.07 | 180.00 |
| G | 60.00 | 36.62 | 180.00 |
| E | 124.62 | -182.00 | 180.00 |
| |
|
| Phe | |
| A | -62.99 | -44.51 | 180.00 |
| C | -91.84 | 1.34 | 180.00 |
| B | -125.17 | 137.46 | 180.00 |
| P | -72.45 | 134.57 | 180.00 |
| G | 61.78 | 30.44 | 180.00 |
| E | 179.00 | -202.00 | 180.00 |
| |
|
| Gly | |
| A | -65.67 | -43.68 | 180.00 |
| C | -85.77 | 1.73 | 180.00 |
| B | -134.58 | 167.71 | 180.00 |
| P | -72.89 | 157.18 | 180.00 |
| G | 85.44 | 9.80 | 180.00 |
| E | 105.54 | -171.71 | 180.00 |
| O | -89.00 | 210.00 | 0.00 |
|
| His | |
| A | -67.48 | -42.86 | 180.00 |
| C | -96.70 | -0.65 | 180.00 |
| B | -129.14 | 137.86 | 180.00 |
| P | -69.71 | 134.71 | 180.00 |
| G | 59.38 | 39.08 | 180.00 |
| E | 132.67 | -215.67 | 180.00 |
| |
|
| Ile | |
| A | -65.19 | -44.98 | 180.00 |
| C | -88.15 | -5.38 | 180.00 |
| B | -118.70 | 132.65 | 180.00 |
| P | -73.39 | 129.79 | 180.00 |
| E | 72.50 | -168.00 | 180.00 |
| |
| |
|
| Lys | |
| A | -64.89 | -41.63 | 180.00 |
| C | -85.39 | -6.48 | 180.00 |
| B | -119.89 | 141.30 | 180.00 |
| P | -70.78 | 133.27 | 180.00 |
| G | 65.94 | 34.94 | 180.00 |
| E | 110.83 | -174.25 | 180.00 |
| |
|
| Leu | |
| A | -65.39 | -41.96 | 180.00 |
| C | -85.39 | -5.06 | 180.00 |
| B | -117.18 | 134.20 | 180.00 |
| P | -72.69 | 135.93 | 180.00 |
| G | 74.38 | 36.85 | 180.00 |
| E | 80.50 | -194.50 | 180.00 |
| |
|
| Met | |
| A | -66.15 | -40.24 | 180.00 |
| C | -88.04 | -4.36 | 180.00 |
| B | -126.36 | 138.63 | 180.00 |
| P | -68.93 | 130.93 | 180.00 |
| G | 48.75 | 42.50 | 180.00 |
| E | 114.00 | -189.00 | 180.00 |
| |
|
| Asn | |
| A | -65.79 | -41.91 | 180.00 |
| C | -95.82 | 5.26 | 180.00 |
| B | -121.34 | 131.40 | 180.00 |
| P | -73.60 | 130.31 | 180.00 |
| G | 60.05 | 34.44 | 180.00 |
| E | 79.14 | -180.71 | 180.00 |
| |
|
| Pro | |
| A | -55.68 | -38.42 | 180.00 |
| C | -67.59 | -9.61 | 180.00 |
| B | -106.20 | 148.80 | 180.00 |
| P | -63.43 | 143.52 | 180.00 |
| G | 106.00 | 65.00 | 180.00 |
| O | -78.57 | 127.26 | 0.00 |
| |
|
| Gln | |
| A | -65.41 | -40.70 | 180.00 |
| C | -85.21 | -6.51 | 180.00 |
| B | -122.35 | 142.72 | 180.00 |
| P | -72.20 | 137.59 | 180.00 |
| G | 56.69 | 39.62 | 180.00 |
| E | 80.25 | -206.75 | 180.00 |
| |
|
| Arg | |
| A | -64.11 | -42.92 | 180.00 |
| C | -88.39 | -5.79 | 180.00 |
| B | -126.38 | 133.86 | 180.00 |
| P | -68.87 | 133.18 | 180.00 |
| G | 60.36 | 40.79 | 180.00 |
| E | 92.80 | -157.80 | 180.00 |
| |
|
| Ser | |
| A | -64.87 | -40.48 | 180.00 |
| C | -85.17 | -4.96 | 180.00 |
| B | -127.22 | 146.43 | 180.00 |
| P | -70.17 | 143.72 | 180.00 |
| G | 74.53 | 27.80 | 180.00 |
| E | 108.93 | -174.71 | 180.00 |
| |
|
| Thr | |
| A | -66.17 | -42.24 | 180.00 |
| C | -93.19 | -2.55 | 180.00 |
| B | -120.61 | 144.94 | 180.00 |
| P | -73.22 | 142.94 | 180.00 |
| G | 65.20 | 39.00 | 180.00 |
| E | 132.17 | -163.83 | 180.00 |
| O | -106.00 | 112.00 | 0.00 |
|
| Val | |
| A | -67.46 | -42.91 | 180.00 |
| C | -92.25 | -5.72 | 180.00 |
| B | -119.95 | 133.71 | 180.00 |
| P | -74.31 | 129.00 | 180.00 |
| G | 56.33 | 25.00 | 180.00 |
| E | 129.00 | -221.00 | 180.00 |
| |
|
| Trp | |
| A | -62.96 | -41.89 | 180.00 |
| C | -86.32 | -3.29 | 180.00 |
| B | -125.36 | 145.33 | 180.00 |
| P | -73.91 | 138.36 | 180.00 |
| G | 71.00 | 33.00 | 180.00 |
| |
| |
|
| Tyr | |
| A | -66.11 | -43.75 | 180.00 |
| C | -93.94 | -3.59 | 180.00 |
| B | -125.72 | 140.55 | 180.00 |
| P | -71.79 | 134.35 | 180.00 |
| G | 78.54 | 25.92 | 180.00 |
| E | 174.00 | -187.00 | 180.00 |
| O | -114.50 | 164.00 | 0.00 |
|
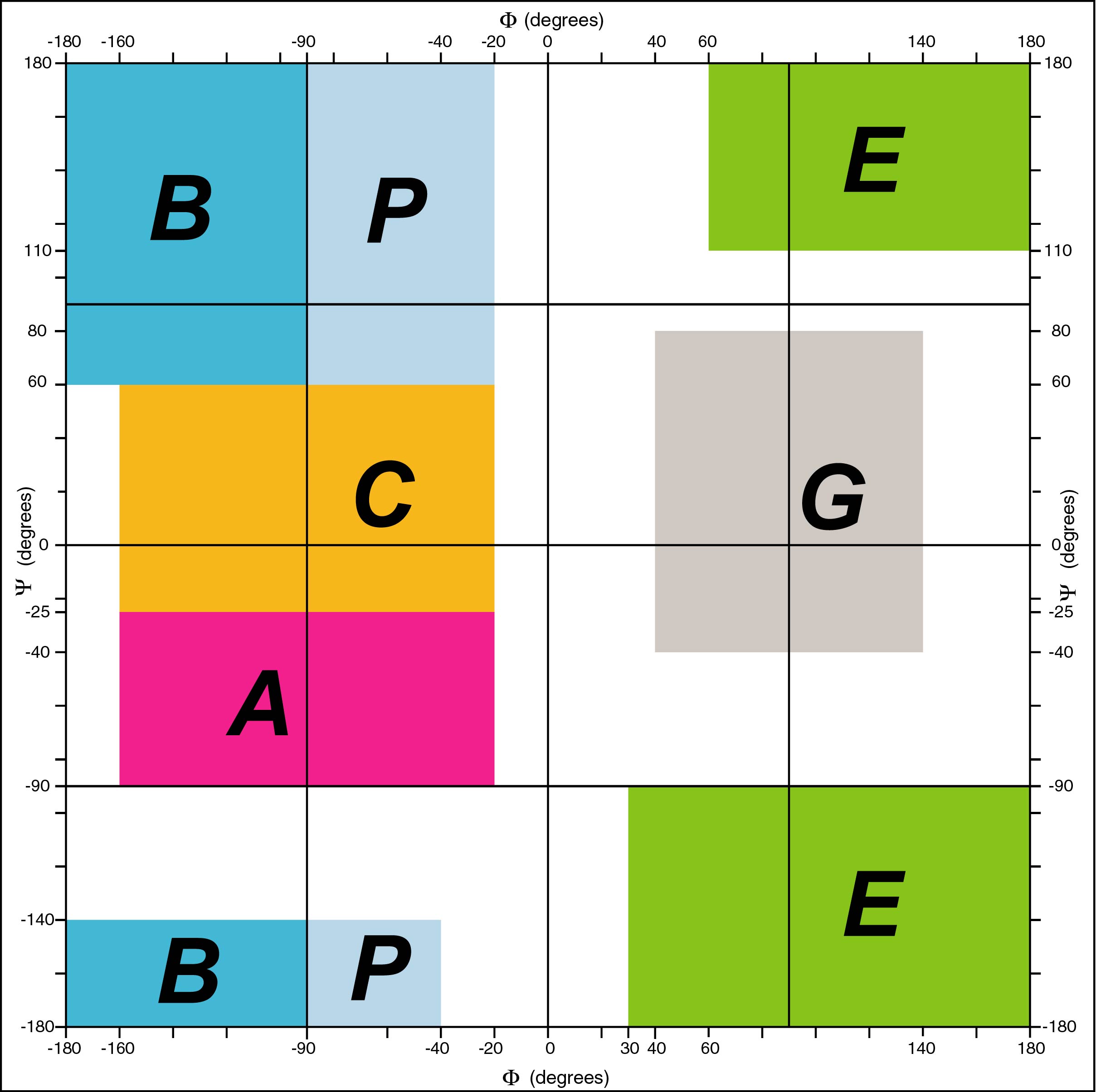 The backbone conformation of a protein is represented by the (phi , psi,
omega) torsion angles of its residues. These angles are grouped into seven
domains called A, C, B, P, G, E and O. Domain O (not shown) groups all
cis-conformations (omega = 0°). The other six domains correspond to
trans-conformations (omega = 180°); A groups alpha-helical and C
3-10-helical structures, B corresponds to beta-like and P to
polyproline-like extended
conformations and G and E have negative phi angles, mirror-symetrical with
A/C and B/P, respectively.
The backbone conformation of a protein is represented by the (phi , psi,
omega) torsion angles of its residues. These angles are grouped into seven
domains called A, C, B, P, G, E and O. Domain O (not shown) groups all
cis-conformations (omega = 0°). The other six domains correspond to
trans-conformations (omega = 180°); A groups alpha-helical and C
3-10-helical structures, B corresponds to beta-like and P to
polyproline-like extended
conformations and G and E have negative phi angles, mirror-symetrical with
A/C and B/P, respectively.